Enbrel Injection Uses, Dosage, Side Effects, Precautions
Enbrel Injection >> Generic drug of the therapeutic class: Immunology
active ingredients: Etanercept
Table of Contents
what is enbrel?
- Etanercept suppresses body defenses and inhibits inflammation.
- In joint inflammation (rheumatoid arthritis, Bechterew’s disease) and psoriasis (scaling skin disease). Sometimes also with inflammatory bowel disease (Crohn’s disease) and Wegener’s disease (chronic vascular inflammation).
- You will usually notice that your illness will become calmer within a few weeks.
- You can inject etanercept yourself. A nurse will explain how you do that.
- Usually you use the injection twice a week, sometimes once.
- Redness, itching and swelling at the injection site occur regularly. Therefore, always choose another injection site for example in the thigh, abdomen or back of the upper arm. Usually this local update takes 3 to 5 days. After a month you are less affected by this.
- You are more likely to have infections such as colds, bronchitis and forehead inflammation. This is because the body’s defense is reduced. Always consult your doctor if you have a fever.
what is enbrel injection used for and indication?
Rheumatoid arthritis :
- Enbrel Injection in combination with methotrexate is indicated for the treatment of moderate to severely active rheumatoid arthritis in adults with inadequate response to DMARDs, including methotrexate (unless contraindicated).
- Enbrel Injection may be given as monotherapy in case of intolerance to methotrexate or when continued treatment with methotrexate is unsuitable.
- Enbrel Injection is also indicated for the treatment of severe, active and progressive rheumatoid arthritis in adults not previously treated with methotrexate.
- Enbrel Injection, alone or in combination with methotrexate, has been shown to slow the progression of structural joint damage as measured by X-ray and improve functional abilities.
Juvenile idiopathic arthritis :
- Treatment of rheumatoid arthritis (positive or negative rheumatoid factor) and extensive oligoarthritis in children from 2 years of age and adolescents in case of inadequate response or proven intolerance to methotrexate.
- Treatment of psoriatic arthritis of the adolescent from the age of 12 years in case of inadequate response or proven intolerance to methotrexate.
- Treatment of enthesitis-related arthritis in adolescents from the age of 12 years in the event of an inadequate response or proven intolerance to the reference treatment.
- Enbrel Injection has not been studied in children under 2 years old.
Psoriatic arthritis
- Treatment of active and progressive psoriatic arthritis of the adult in case of inadequate response to previous background therapy.
- Enbrel Injection has been shown to improve functional abilities in patients with psoriatic arthritis, and to slow the progression of peripheral joint structural damage as measured by radiography in patients with symmetrical polyarticular forms of the disease.
Axial spondyloarthritis
Ankylosing spondylitis (AS)
- Treatment of severe and active ankylosing spondylitis in adults with inadequate response to conventional therapy.
Non-radiographic axial spondyloarthritis
Treatment of severe non-radiographic axial spondyloarthritis of the adult with objective signs of inflammation, resulting in high levels of C-reactive protein (CRP) and / or visible signs on magnetic resonance imaging (MRI), in case of inadequate response to nonsteroidal anti-inflammatory drugs (NSAIDs).
Plaque psoriasis
- Treatment of moderate-to-severe plaque psoriasis in adults in the event of failure, contra-indication, or intolerance to other systemic treatments including ciclosporin, methotrexate or PUVA.
Psoriasis in plaques of the child
- Treatment of severe chronic plaque psoriasis in children from 6 years of age and adolescents in case of inadequate control, or intolerance to other systemic treatments or phototherapy.
enbrel dosage
Treatment with Enbrel Injection must be initiated and supervised by a medical specialist experienced in the diagnosis and treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, plaque psoriasis or plaque psoriasis in the child. The Patient Monitor Card should be given to patients treated with Enbrel Injection.
Enbrel Injection is available in dosages of 10, 25 and 50 mg.
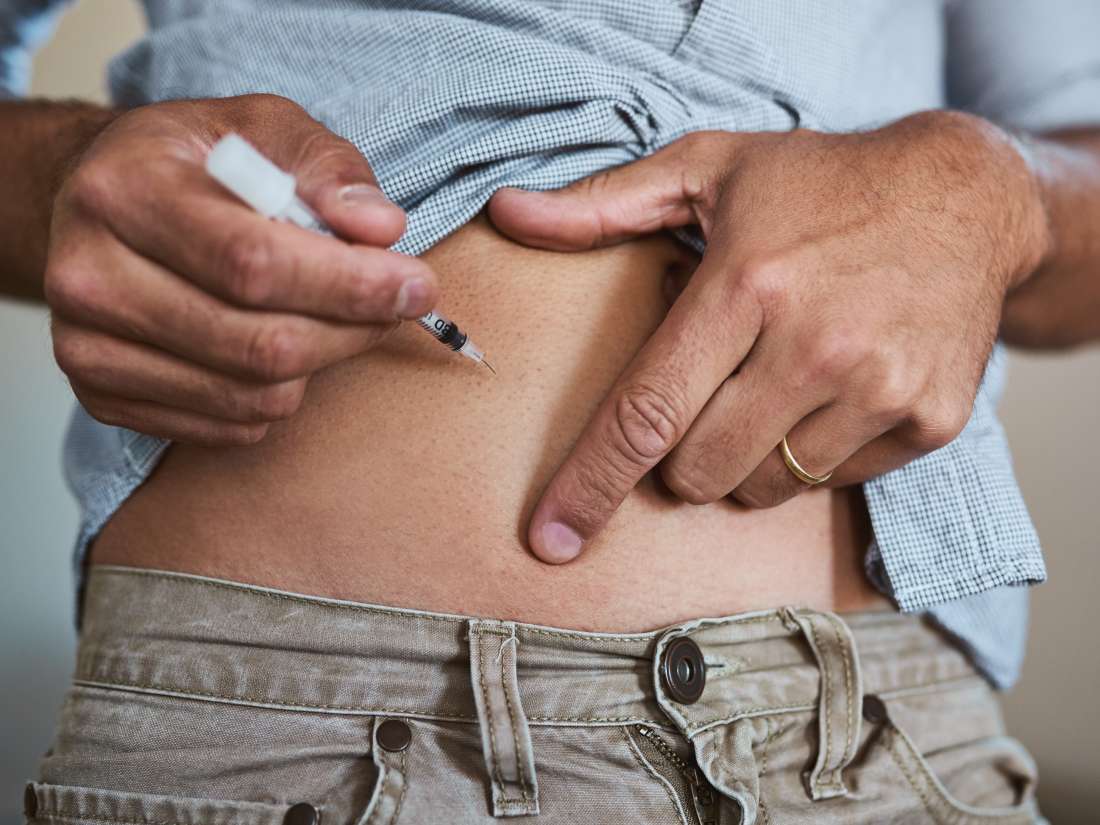
Dosage
Rheumatoid arthritis :
- The recommended dose of Enbrel Injection is 25 mg twice weekly. However, the efficacy and safety of administration of 50 mg once a week has been demonstrated .
- Psoriatic arthritis, ankylosing spondylitis and non-radiographic axial spondyloarthritis The recommended dose is 25 mg Enbrel Injection twice weekly or 50 mg once weekly.
- For all the above indications, the available data suggest that a clinical response is usually obtained within 12 weeks of treatment. Further treatment should be carefully reconsidered in a patient who has not responded within this time frame.
Plaque psoriasis :
- The recommended dose of Enbrel Injection is 25 mg twice daily or 50 mg once weekly. However, a dose of 50 mg twice a week may be used up to 12 weeks, followed, if necessary, by a dose of 25 mg twice a week or 50 mg once a week. Treatment with Enbrel Injection should be continued until remission is achieved, up to a maximum of 24 weeks. Continuous treatment beyond 24 weeks may be appropriate for some adult patients ( Pharmacodynamic properties ).
- Treatment with Enbrel Injection should be discontinued in patients with no response after 12 weeks of treatment.
- If re-treatment with Enbrel Injection is indicated, the same treatment duration schedule should be followed. The dose should be 25 mg given twice a week or 50 mg once a week.
Special populations
Patients with renal and hepatic impairment
- No dosage adjustment is necessary.
Elderly patients
- No dosage adjustment is necessary. The dosage and administration are identical to those of adults aged 18 to 64 years.
Pediatric population :
- The dose of Enbrel Injection depends on the weight of pediatric patients. Patients weighing less than 62.5 kg should be given an exact dose calculated in mg / kg using the powder and solvent for solution for injection or the powder for solution for injection (see below for doses as indicated). Patients weighing 62.5 kg or more can receive a fixed dose using the pre-filled syringe or pre-filled pen (50 mg / week).
Juvenile idiopathic arthritis :
- The recommended dose is 0.4 mg / kg (maximum 25 mg per injection) administered twice a week by subcutaneous injection, with an interval of 3-4 days between two injections or 0.8 mg / kg ( maximum 50 mg per injection) administered once a week. Discontinuation of treatment should be considered in non-responder patients after 4 months.
- The 10 mg dosage is more suitable for administration to children with JIA less than 25 kg.
- No clinical trials have been conducted in children aged 2 to 3 years. Limited safety data from a patient registry, however, suggests that the safety profile in children aged 2 to 3 years is similar to that of adults and children over 4 years of age, at a dose of 0, 8 mg / kg subcutaneously weekly (see section 5.1 Pharmacodynamic properties ).
- Enbrel Injection is generally not recommended for use in children under 2 years of age for juvenile idiopathic arthritis.
Plaque psoriasis of the child (6 years old and over)
- The recommended dose is 0.8 mg / kg (maximum 50 mg per injection) once a week up to 24 weeks. Treatment should be discontinued in patients who do not respond after 12 weeks of treatment.
- If resumption of treatment with Enbrel Injection is indicated, the treatment duration schedule described above should be followed. The dose should be 0.8 mg / kg (maximum 50 mg per injection) once a week.
- Enbrel Injection is generally not recommended for use in children under 6 years of age for plaque psoriasis.
- Full instructions for administration are given in the package leaflet, section 7 “Instructions for the preparation and administration of an injection of Enbrel Injection”.
Contraindications
- Etanercept hypersensitivity
- Septicemia
- Infection
- Child under 2 years old
- Lack of effective female contraception
- Feeding with milk
- Moderate to severe alcoholic hepatitis
- Pregnancy
Hypersensitivity to the active substance or to any of the excipients listed in the Composition section. Sepsis or risk of sepsis.
Treatment with Enbrel should not be initiated in patients with active infection including chronic or localized infections.
how enbrel works?
Pharmacotherapeutic group: immunosuppressive agents, inhibitors Necrosis Factor alpha Tumors (TNF α ), ATC code: L04AB01
Tumor necrosis factor (TNF) is a dominant cytokine in the inflammatory process of rheumatoid arthritis. High levels of TNF are also found in the synovial membranes and psoriatic plaques of patients with psoriatic arthritis, and in the serum and synovial tissue of patients with ankylosing spondylitis. In psoriasis plaques, infiltration by inflammatory cells, including T cells, leads to an increase in TNF levels in psoriatic lesions, compared to levels seen in non-skin areas. Etanercept is a competitive inhibitor of TNF binding to its surface receptors thereby inhibiting the biological activity of TNF.
TNF and lymphotoxin are pro-inflammatory cytokines that bind two distinct receptors on the cell surface: tumor necrosis factor receptors (TNFRs) of 55-kilodalton (p55) and 75-kilodalton (p75). These two TNFRs naturally exist in membrane and soluble forms. Soluble TNFRs are thought to regulate the biological activity of TNF.
TNF and lymphotoxin exist mainly as homotrimers, their biological activity being dependent on the TNFR crosslinking at the cell surface. Soluble dimeric receptors such as etanercept have a greater affinity for TNF than monomeric receptors and are much more potent competitive inhibitors of TNF binding to its cellular receptors. In addition, the use of an immunoglobulin Fc region as a fusion element in the construction of a dimeric receptor confers on the molecule a longer plasma half-life.
Action mechanism
The majority of joint damage in rheumatoid arthritis and ankylosing spondylitis, and skin involvement of plaque psoriasis is mediated by pro-inflammatory molecules that belong to a TNF-controlled network. The supposed mechanism of action of etanercept is competitive inhibition of TNF binding to TNFR of the cell surface: TNF-mediated cellular responses are blocked by rendering TNF biologically inactive. Etanercept may also modulate the biological responses controlled by other molecules acting downstream (eg cytokines, adhesins or proteinases) whose activity is induced or regulated by TNF.
Clinical efficacy and safety
This section presents data from four randomized controlled trials in adults with rheumatoid arthritis, a study in adults with psoriatic arthritis, a study in adults with ankylosing spondylitis, a study in adults with non-radiographic axial spondyloarthritis, four studies in adults with plaque psoriasis, three studies in juvenile idiopathic arthritis, and one study in children with plaque psoriasis.
Adult patients with rheumatoid arthritis
The efficacy of Enbrel Injection was evaluated in a randomized, double-blind, placebo-controlled study. The study evaluated 234 adult patients with active rheumatoid arthritis who did not respond to at least one and at most four DMARDs. Doses of 10 mg or 25 mg of Enbrel Injection or placebo were administered subcutaneously twice a week for 6 consecutive months. The results of this controlled study were expressed as percentage improvement in rheumatoid arthritis, using the American College of Rheumatology (ACR) response criteria.
ACR 20 and ACR 50 responses were higher in Enbrel Injection-treated patients compared to placebo at 3 and 6 months (ACR 20: Enbrel Injection 62% and 59%, placebo 23% and 11% at 3 and 6 months respectively; ACR 50 : Enbrel Injection 41% and 40%, placebo 8% and 5% respectively at 3 and 6 months, p <0.01 Enbrel Injection vs placebo at all measurement times for the ACR 20 and ACR 50 responses).
Approximately 15% of patients receiving Enbrel Injection achieved an ACR 70 response at 3 months and at 6 months compared to less than 5% of placebo-treated patients. Among patients receiving Enbrel Injection, clinical responses generally started 1 to 2 weeks after initiation of treatment, and were almost always achieved within 3 months. A dose-dependent response was observed; results with 10 mg were intermediate between placebo and 25 mg. Enbrel Injection was significantly superior to placebo on all ACR criteria items, as well as other measures of rheumatoid arthritis activity not included in these ACR response criteria, such as duration of morning stiffness. The Health Assessment Questionnaire (HAQ) scale, including disability, activity, mental state, general condition, state of joint function, was evaluated every 3 months during the study. All areas of the HAQ scale were improved in patients treated with Enbrel Injection compared to placebo-treated patients at 3 and 6 months.
After the arrest of Enbrel Injection, arthritis symptoms usually reappeared in the following month. Based on the results of the open label studies, restarting treatment with Enbrel Injection after periods of up to 24 months resulted in the same range of response as in patients receiving Enbrel Injection without interruption of treatment. Stable and lasting responses have been observed in patients receiving Enbrel Injection continuously up to 10 years in open-label studies (extension phase of therapeutic studies).
The efficacy of Enbrel Injection was compared with methotrexate in a randomized, controlled-active study with blinded radiographic endpoints as a primary endpoint in 632 adult patients with active rheumatoid arthritis (<3 years duration). ) who had never received treatment with methotrexate. Enbrel Injection 10 mg or 25 mg doses were administered subcutaneously twice a week for up to 24 months. Methotrexate doses were increased from 7.5 mg / week to 20 mg / week maximum during the first 8 weeks of the trial and maintained for up to 24 months. With Enbrel Injection 25 mg clinical improvement, including the onset of action within two weeks, was similar to that seen in previous trials, and is maintained for up to 24 months. At inclusion, patients had a moderate degree of disability, with mean HAQ scores of 1.4 to 1.5. Treatment with Enbrel Injection 25 mg resulted in a significant improvement at 12 months, with approximately 44% of patients achieving a normal HAQ score (less than 0.5). This benefit was maintained in the second year of this study.
In this study, structural joint damage was assessed radiographically and expressed as a modification of the Sharp Total Score (STS) and its components; Erosion score and Articular Pinching Score (APS). X-rays of the hands / wrists and feet were read at inclusion and at 6, 12 and 24 months. The 10 mg dose of Enbrel Injection had consistently less effect on structural damage than the 25 mg dose. Enbrel Injection at 25 mg was significantly superior to methotrexate for erosion scores, at both 12 and 24 months. Differences between the methotrexate group and the 25 mg Enbrel Injection group for STS and SPA were not statistically significant.
In another controlled versus active, randomized, double-blind study, clinical efficacy, tolerability, and radiographic outcome in patients with RA treated with Enbrel Injection alone (25 mg twice weekly), or methotrexate alone (7.5 to 20 mg weekly, 20 mg median dose) or concurrently initiated Enbrel Injection plus methotrexate, were compared in 682 adult patients with active rheumatoid arthritis of 6 months to 20 years (median 5 years) and who had an inadequate response to at least one DMARD other than methotrexate.
Patients treated with Enbrel Injection plus methotrexate had ACR 20, ACR 50, and ACR 70 responses, and significantly improved DAS and HAQ scores at both 24 and 52 weeks, compared with patients in each of the monotherapy groups. (results presented in the table below). Significant benefits with Enbrel Injection plus methotrexate compared with Enbrel Injection monotherapy and methotrexate monotherapy were also observed after 24 months.
what are the enbrel side effects?
In addition to the desired effect, this can cause drug side effects.
The main side effects are the following.
Regularly (with more than 30 in 100 people)
- Redness, itching and swelling at the site of injection. These symptoms usually last 3 to 5 days. You are particularly troubled by the first month. Thereafter, this side effect is usually less.
Sometimes (from 10 to 30 people in 100)
- More risk of infections , such as colds, bronchitis, forehead inflammation, bladder infections or skin infections. Also tuberculosis can rarely occur.
- Infections arise earlier because the body’s immune system is reduced. If you get a fever or signs of an infection, always consult your doctor. Rarely, a serious infection develops in the blood; this can sometimes only become noticeable after a few weeks of use.
- Childrenthose treated with etanercept may be more likely to develop a childhood disease, such as chickenpox. Moreover, the childhood disease can be more serious. For children who have not yet received all vaccinations against childhood diseases, it is better to first get all vaccinations before they start etanercept.
Consult with the doctor about this.
Rarely (from 1 to 10 in 100 people)
- Hypersensitivity to this medication. You will notice this by skin rash, itching, hives, fever, tightness or fainting. Then warn a doctor.
- In rare cases ‘angioedema’ develops: a swelling of the face, lips, mouth, tongue or throat. You can be very stuffy. If it occurs, you should immediately seek out a doctor or go to the First Aid Service.
- In very rare cases a severe skin condition can develop with blistering. The blisters mainly develop on the lips and on the mucous membranes of the mouth and genitals. Then contact a doctor immediately.
- If you are hypersensitive you should not use this medicine in the future.
- Therefore tell the pharmacist that you are hypersensitive to etanercept.
- The pharmacy team can then ensure that you do not get this medication again.
Very rare (affects less than 1 in 100 people)
- Worsening of psoriasis . Consult your doctor.
- Less red and white blood cells or platelets. This can occur after a few weeks or months. Your doctor will therefore have your blood checked regularly. You notice extreme fatigue, sore throat, fever, blisters in the mouth, bruises or bloody noses and more chance of infections. In these cases, tell your doctor immediately.
- Heart failure or more heart failure. In the event of increased fluid retention (thick ankles) or tightness, you should warn your doctor.
- More risk of chronic diseases , such as lupus erythematodes (LE), blood vessel inflammation, inflamed muscles and skin (dermatomyositis), organ inflammation (sarcoidosis), liver inflammation (hepatitis), intestinal inflammation and ocular inflammation (uveitis).
- More risk of certain types of cancer , such as lymphoma , blood cancer (leukemia), skin cancer, breast cancer or lung cancer.
- Nervous abnormalities . In people with multiple sclerosis (MS) or optic nerve abnormalities, the symptoms can worsen.
- People who have had hepatitis B before may be given this again. In people with h epatitis C the symptoms may worsen. Consult your doctor.
Consult your doctor if you suffer too much from one of the above mentioned side effects or if you experience other side effects that you are worried about.
Enbrel Injection Interactions
Concomitant treatment with anakinra

- Adult patients treated with Enbrel Injection and anakinra had a higher rate of serious infections compared to patients treated with either Enbrel Injection alone or anakinra alone (historical data).
- In addition, in a double-blind, placebo-controlled trial in adult patients receiving methotrexate DMARD, patients treated with Enbrel Injection and anakinra experienced a higher rate of serious infections (7%). ) and neutropenia as patients treated with Enbrel Injection alone (see Warnings and Precautions and Adverse Reactions sections ). The combination of Enbrel Injection and anakinra has not demonstrated superior clinical benefit and is therefore not recommended.
Concomitant treatment with abatacept
- Concomitant administration of abatacept and Enbrel Injection in clinical studies has resulted in an increased incidence of serious adverse events.
- This association did not demonstrate additional clinical benefit; therefore this combination is not recommended (see Warnings and Precautions section ).
Concomitant treatment with sulfasalazine
- In a clinical study in adult patients treated with stable doses of sulfasalazine and in which Enbrel Injection was added, patients in the group receiving this combination showed a significant decrease in mean white blood cell count, compared to the groups treated with Enbrel Injection or sulfasalazine alone.
- The clinical significance of this interaction is unknown.
- The use of Enbrel Injection in combination with sulfasalazine should be considered with caution.
No interactions
- In clinical trials, no interaction was observed when Enbrel Injection was administered with glucocorticoids, salicylates (except sulfasalazine), nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics or methotrexate. See Warnings and Precautions for Immunization Recommendations.
- No significant pharmacokinetic drug interaction was observed in the methotrexate, digoxin and warfarin studies.
Warnings and Precautions
- Infections should be investigated in patients before, during, and after treatment with Enbrel Injection, taking into account that the mean elimination half-life of etanercept is approximately 70 hours (between 7 and 300 hours).
- Serious infections, septicemia, tuberculosis, and opportunistic infections, including invasive fungal infections, listeriosis and legionellosis, have been reported with Enbrel Injection (see section 4.8).). These infections were caused by bacteria, mycobacteria, fungi, viruses and parasites (including protozoa). In some cases, specific fungal infections and other opportunistic infections have not been diagnosed, resulting in delayed initiation of appropriate treatment and sometimes death. When assessing the risk of infection in a patient, exposure to risk factors specifically associated with certain opportunistic infections (eg exposure to endemic fungal infections) should be assessed and considered.
- Careful monitoring should be exercised in patients treated with Enbrel Injection developing a new infection. Treatment with Enbrel Injection should be discontinued if the patient develops a serious infection. The safety and efficacy of Enbrel Injection in patients with chronic infections have not been evaluated. Physicians should prescribe Enbrel Injection with caution to patients with a history of recurrent or chronic infections, or having a predisposing course for infections such as severe or poorly balanced diabetes.
Tuberculosis
- Cases of active tuberculosis including miliary tuberculosis and tuberculosis with extra-pulmonary localization have been reported in patients treated with Enbrel Injection.
- Before initiating treatment with Enbrel Injection, a search for active or inactive (“latent”) TB should be performed in all patients. This research should include a detailed medical interview on the personal history of tuberculosis or any previous contacts with a TB patient and on previous and / or ongoing immunosuppressive therapy. Appropriate screening tests, such as a dermal tuberculin test and a chest X-ray, should be performed in all patients (in accordance with local recommendations). It is recommended that you write these exams on the Patient Monitor Card. Prescribers are reminded that dermal tuberculin testing may be falsely negative, particularly in a severely ill or immunocompromised patient.
- If active TB is diagnosed, treatment with Enbrel Injection should not be initiated. In the case of diagnosis of inactive (“latent”) TB, appropriate prophylactic TB treatment should be initiated prior to initiating Enbrel Injection, and in accordance with recommendations.
- local. In such a case, the benefit / risk ratio of treatment with Enbrel Injection should be carefully evaluated.
- All patients should be made aware of the need to consult a physician if signs or symptoms suggestive of TB (eg, persistent cough, weight loss / weight loss, fever) occur during or after treatment with Enbrel Injection.
Reactivation of hepatitis B
- Hepatitis B reactivation has been reported in patients previously infected with the hepatitis B virus (HBV) and treated with anti-TNF, including Enbrel Injection. This includes cases of reactivation of hepatitis B in patients positive for anti-HBc but negative for HBs.
- Patients should be screened for HBV infection before initiating treatment with Enbrel Injection.
- If the results of the screening are positive, it is recommended to consult a doctor specialized in the treatment of hepatitis B; precautions to be taken when administering Enbrel Injection to patients previously infected with HBV.
- In these patients, the signs and symptoms of active infection with HBV throughout the course of treatment and for several weeks after the end of treatment.
- No relevant data for treating HBV-positive patients with anti-TNF-related antiviral therapy is available. In patients who develop HBV infection, treatment with Enbrel Injection should be discontinued and effective antiviral therapy with symptomatic treatment should be initiated.
Aggravation of hepatitis C
- Cases of worsening hepatitis C have been reported in patients receiving Enbrel Injection.
- Enbrel Injection should be used with caution in patients with a history of hepatitis C.
Concomitant treatment with anakinra
- Concomitant administration of Enbrel Injection and anakinra has been associated with an increased risk of serious infections and neutropenia compared with Enbrel Injection when administered alone.
- This association has not demonstrated superior clinical benefit.
- Therefore the combination of Enbrel Injection and anakinra is not recommended (see sections Interactions with other medicinal products and other forms of interaction and side effects ).
Concomitant treatment with abatacept
- Concomitant administration of abatacept and Enbrel Injection in clinical studies has resulted in an increased incidence of serious adverse events.
- This association did not demonstrate additional clinical benefit; therefore this combination is not recommended (see section Interactions with other medicinal products and other forms of interaction ).
Allergic reactions
- Allergic reactions associated with the administration of Enbrel Injection have been frequently reported. These allergic reactions included cases of angioedema and urticaria; serious reactions have occurred. In case of severe allergic reaction or anaphylactic reaction, treatment with Enbrel Injection should be discontinued immediately and appropriate treatment instituted.
- The needle guard of the pre-filled syringe contains latex (natural rubber) that can cause hypersensitivity reactions when handled or when Enbrel Injection is administered in persons with known or potential latex sensitivity.
immunosuppression
- Anti-TNFs, including Enbrel Injection, may alter the patient’s immune defenses against infections and malignancies, especially as TNF mediates inflammation and modulates the immune response. cells. In a study of 49 adult patients with rheumatoid arthritis treated with Enbrel Injection, no reduction in delayed hypersensitivity, immunoglobulin levels, or changes in blood count was observed.
- Two patients with juvenile idiopathic arthritis developed chickenpox with signs and symptoms of aseptic meningitis followed by a cure without sequelae. Patients exposed to the chickenpox virus should temporarily discontinue Enbrel Injection and prophylaxis with specific immunoglobulins should be considered.
- The safety and efficacy of Enbrel Injection in immunocompromised patients have not been evaluated.
Malignant neoplasms and lymphoproliferative disorders
Solid tumors and hematopoietic disorders (excluding skin cancer)
- Various cases of malignant tumors (breast cancer, lung cancer, lymphoma) have been reported after marketing.
- In the controlled phases of clinical trials with anti-TNF, more cases of lymphoma were observed in patients who received anti-TNF than in control patients. However, the occurrence was rare and the follow-up period for patients on placebo was shorter than that for patients receiving anti-TNF therapy. After marketing, cases of leukemia have been reported in patients treated with anti-TNF. There is an increased risk of developing lymphoma or leukemia in patients with rheumatoid arthritis when the disease is old, highly active and inflammatory, which complicates the risk assessment.
- In the current state of knowledge, the possibility of developing lymphoma, leukemia or other solid or haematopoietic malignancies in patients treated with anti-TNF can not be ruled out. Precautions should be taken when using anti-TNF therapy in patients with a history of malignancy or when continuing treatment in patients who develop malignancy.
- Malignancies, some of which have been fatal, have been reported post-marketing in children, adolescents and young adults (up to age 22) treated with anti-TNF including Enbrel Injection (initiation of treatment ≤ 18 years). About half of the cases were lymphomas. Other cases were other types of malignancies, including rare malignancies usually associated with immunosuppression. The risk of developing malignant tumors in children and adolescents treated with anti-TNF can not be ruled out.
Skin cancers
- Cases of melanoma and non-melanoma skin cancer have been reported in patients treated with anti-TNF including Enbrel Injection. Cases of Merkel cell carcinoma have been rarely reported after marketing in patients treated with Enbrel Injection. Periodic skin examinations are recommended for all patients, especially those with a risk factor for skin cancer.
- Combining the results of clinical trials, more cases of non-melanoma skin cancer were observed in patients receiving Enbrel Injection compared to the control group, particularly in patients with psoriasis.
immunizations
- Live vaccines should not be administered to patients treated with Enbrel Injection. No data are available on infectious transmission secondary to the administration of live vaccines in patients treated with Enbrel Injection.
- In a randomized, double-blind, placebo-controlled clinical trial in adult patients with psoriatic arthritis, 184 patients also received a multivalent pneumococcal polysaccharide vaccine at week 4.
- In this study, most patients with psoriatic arthritis treated with Enbrel Injection were able to increase the immune response of activated B cells to pneumococcal polysaccharide vaccine; however the aggregates were moderately low and some patients had increased their titre a factor of 2 compared to patients who were not treated with Enbrel Injection.
- The clinical significance of these findings is unknown.
Autoantibody formation
- Enbrel Injection may cause the formation of autoimmune antibodies.
Hematologic reactions
- Rare cases of pancytopenia and very rare cases of bone marrow aplasia, some of which have been fatal, have been reported in patients treated with Enbrel Injection. Special attention should be paid to patients treated with Enbrel Injection who have a history of haematological involvement.
- All patients and parents / relatives should be informed that if signs or symptoms suggestive of hematological involvement or infection (such as persistent fever, pharyngeal pain, bruising, bleeding, pallor) occur in the patient under Enbrel Injection they must immediately consult a doctor.
- In these patients, additional tests, including a blood count, should be performed urgently; if hematological involvement is confirmed, treatment with Enbrel Injection should be discontinued.
Neurological disorders
- Rare cases of CNS demyelinating disorders have been reported in patients treated with Enbrel Injection .
- Very rare cases of demyelinating peripheral polyneuropathies have also been reported (including Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, demyelinating polyneuropathy and multifocal motor neuropathy). Although no clinical trials have been conducted to study Enbrel Injection therapy in multiple sclerosis patients, trials with other TNF blocking agents in patients with multiple sclerosis have shown that an increase in the activity of the disease. It is recommended to carefully evaluate the benefit / risk ratio, with a neurological evaluation before prescribing Enbrel Injection in patients with a history of demyelinating disease or in the event of recent onset of demyelinating disease,
Associated treatment
- In a 2-year controlled clinical trial in patients with rheumatoid arthritis, the combination of Enbrel Injection and methotrexate did not show unexpected safety data, and the safety profile of Enbrel Injection associated with Methotrexate was similar to the profiles reported in studies with Enbrel Injection and methotrexate used alone. Long-term studies evaluating the tolerance of this association are currently underway. The long-term safety of Enbrel Injection in combination with other DMARDs has not been established.
- The use of Enbrel Injection in combination with other systemic treatments or phototherapy in the treatment of psoriasis has not been studied.
Renal and hepatic insufficiency
- Based on pharmacokinetic data , no dose adjustment is required in patients with renal or hepatic impairment; clinical experience in such patients is limited.
Congestive heart failure
- Physicians should use Enbrel Injection with caution in patients with congestive heart failure (CHF). Post-marketing reports of worsening CHF, with or without an identifiable enhancing factor, have been reported in Enbrel Injection patients. There have also been rare cases (<0.1%) of de novo onset of CHF, including in patients with no known cardiovascular history. Some of these patients were under 50 years old. Two large clinical studies evaluating Enbrel Injection in the treatment of CHF were interrupted early due to a lack of efficacy. Although inconclusive, data from one of these studies suggests a possible trend toward worsening CHF, in patients receiving Enbrel Injection.
Alcoholic hepatitis
- In a randomized, placebo-controlled, placebo-controlled Phase II study of 48 hospitalized patients treated with Enbrel Injection or placebo for moderate to severe alcoholic hepatitis, Enbrel Injection was not effective and the mortality rate of patients treated with Enbrel Injection was significantly higher. after 6 months. Therefore, Enbrel Injection should not be used in patients with moderate to severe alcoholic hepatitis.
Wegener granulomatosis
- A placebo-controlled trial, in which 89 adult patients were treated with Enbrel Injection added to standard therapy (including cyclophosphamide or methotrexate, and glucocorticoids) for a median of 25 months, did not demonstrate that Enbrel Injection is a treatment. effective in Wegener granulomatosis. The incidence of non-cutaneous malignancies of different types was significantly higher in patients treated with Enbrel Injection than in the control group. Enbrel Injection is not recommended for the treatment of Wegener’s granulomatosis.
Hypoglycaemia in patients treated for diabetes
- Hypoglycemia has been reported following Enbrel Injection initiation in patients receiving antidiabetic therapy. These hypoglycaemia required a reduction in antidiabetic treatment in some of these patients.
Special populations
Elderly patients
- In phase 3 studies in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis, no overall differences in adverse events, serious adverse events and serious infections were observed in patients with 65 years or older receiving Enbrel Injection compared to younger patients. However, caution should be exercised when treating elderly patients and special attention should be paid to the occurrence of infections.
Pediatric population
immunizations
- It is recommended that children have up-to-date vaccinations, if possible, in accordance with the vaccination schedule in force before initiating treatment with Enbrel Injection (see Vaccinations above).
- Inflammatory bowel disease (IBD) and uveitis in patients with juvenile idiopathic arthritis (JIA)
- Cases of IBD and uveitis have been reported in patients with JIA treated with Enbrel Injection .
Drive and use machines
Pregnancy / Breastfeeding / Fertility
Women of childbearing age
- Women of childbearing potential should be informed that they must use effective contraception to prevent pregnancy during treatment with Enbrel Injection and up to 3 weeks after stopping treatment.
enbrel pregnancy
- Reproductive toxicity studies in rats and rabbits have not shown any damage to the fetus or to the newborn rat due to etanercept.
- A higher rate of major congenital malformations was observed in an observational study comparing pregnancies exposed to etanercept in the first trimester to pregnancies not exposed to etanercept or other TNF-blockers (adjusted odds ratio). 2.4, 95% CI 1.0-5.5).
- The major congenital malformations corresponded to those most frequently encountered in the general population and no particular pattern of abnormality was identified.
- There was no change in the incidence of spontaneous abortions, stillbirths, or minor malformations.
- So,Etanercept crosses the placenta and has been detected in the serum of infants whose mothers had been treated with Enbrel Injection during pregnancy.
- Clinical consequences are not known, but infants may be at increased risk of infection. It is generally not recommended to administer live vaccines to infants up to 16 weeks after the last dose of Enbrel Injection received by the mother.
feeding
- It has been reported that following subcutaneous administration, etanercept was excreted in human breast milk.
- In lactating rats, after subcutaneous administration, etanercept was excreted in milk and detected in serum of neonates.
- Because immunoglobulins, like many drugs, can be excreted in breast milk, it must be decided either to discontinue breastfeeding or to discontinue treatment with Enbrel Injection, taking into account the benefit of breastfeeding for breastfeeding.
- child and the benefit of treatment for the woman.
Fertility
- There are no preclinical data available on the peri- and postnatal toxicity of etanercept or on the effects of etanercept on fertility and overall reproductive function.
What should I do if I miss a dose?
It is important to use this medication consistently. If you have forgotten a dose:
Does it take longer than 24 hours before you should use the next dose?
- Use the missed dose immediately.
- The next dose you can simply administer at the usual time the day after.
Does it take less than 24 hours before you should use the next dose?
- Then talk to your doctor. Sometimes you have to skip the missed dose.
- Sometimes you should use the missed dose and postpone the next dose for a day.
- Never use a double dose in one day.
What happens if I overdose from Enbrel Injection ?
- No toxic dose limits were observed during clinical trials in patients with rheumatoid arthritis. The highest dose tested was an intravenous challenge dose of 32 mg / m 2 , followed by subcutaneous doses of 16 mg / m 2administered twice weekly.
- One patient with rheumatoid arthritis mistakenly self-administered Enbrel Injection 62 mg subcutaneously twice weekly for three weeks without adverse effects.
- There is no known antidote for Enbrel Injection.
What is Forms and Composition?
Solution for injection SC (clear, colorless or pale yellow) 25 mg and 50 mg: Pack of 4 pre-filled syringes * + 4 alcoholic buffers.
* The needle guard contains natural rubber (latex): see Warnings and Precautions for use . Solution for injection SC (clear, colorless or pale yellow) 50 mg: Pack of 4 pre-filled pens (Myclic) * + 4 alcoholic tampons. * The pen cap contains natural rubber (latex): see Warnings and Precautions for use . Powder (white) and solvent (clear and colorless) for 10 mg SC injection for pediatric use:
Box of 4 vials of powder + 4 syringes pre-filled with solvent (water ppi) + 4 needles + 4 adapters for vial + 8 alcohol swabs.
Powder (white) and solvent (clear and colorless) for solution for injection SC 25 mg: Set of 4 vials of powder + 4 pre-filled syringes of solvent (water ppi) + 4 needles + 4 adapters for vial + 8 alcohol swabs.
NOT’s
Edrug-online contains comprehensive and detailed information about drugs available in the medical field, and is divided into four sections:
general information:
- Includes a general description of the drug, its use, brand names, FAQs, and relevant news and articles
Additional information:
- General explanation about dealing with the medicine: how to take the medicine, the doses and times of it, the start and duration of its effectiveness, the recommended diet during the period of taking the medicine, the method of storage and storage, recommendations in cases for forgetting the dose and instructions to stop taking the drug and take additional doses.
Special warnings:
- For pregnant and breastfeeding women, the elderly, boys and drivers, and use before surgery.
Side effects:
- It treats possible side effects and drug interactions that require attention and its effect on continuous use.
- The information contained in this medicine is based on medical literature, but it is not a substitute for consulting a doctor.

Hello. I have checked your edrug-online.com and i see you’ve got some duplicate content so probably it is the reason that
you don’t rank hi in google. But you can fix this issue fast.
There is a tool that creates content like human, just search in google: miftolo’s tools
Hi. I have checked your edrug-online.com and i see you’ve got some duplicate content so probably it is the reason that you don’t
rank high in google. But you can fix this issue fast.
There is a tool that rewrites content like human, just search in google: miftolo’s tools
50 Discount For All Private Proxies! Elite quality, Infinite bandwidth, 1000 mb/s superspeed, 99,9 uptime, Low successive IP’s, Number consumption restrictions, Multiple subnets, USA or Europe proxies – Get Today –
Lovely site! I am loving it!! Will be back later to read some more. I am bookmarking your feeds also.
Hello. I have checked your edrug-online.com and i see you’ve got some duplicate content so
probably it is the reason that you don’t rank hi in google.
But you can fix this issue fast. There is a
tool that rewrites articles like human, just search in google: miftolo’s tools
[…] Enbrel Injection Uses, Dosage, Side Effects, Precautions […]